Pre Gene Modal
BGIBMGA013297
Annotation
PREDICTED:_ryanodine_receptor_44F_isoform_X18_[Bombyx_mori]
Full name
Ryanodine receptor
+ More
Ryanodine receptor 2
Ryanodine receptor 3
Ryanodine receptor 1
Ryanodine receptor 2
Ryanodine receptor 3
Ryanodine receptor 1
Alternative Name
Ryanodine receptor 44F
Cardiac muscle ryanodine receptor
Cardiac muscle ryanodine receptor-calcium release channel
Type 2 ryanodine receptor
Cardiac muscle-type ryanodine receptor
Brain ryanodine receptor-calcium release channel
Brain-type ryanodine receptor
Type 3 ryanodine receptor
Skeletal muscle calcium release channel
Skeletal muscle ryanodine receptor
Skeletal muscle-type ryanodine receptor
Type 1 ryanodine receptor
Cardiac muscle ryanodine receptor
Cardiac muscle ryanodine receptor-calcium release channel
Type 2 ryanodine receptor
Cardiac muscle-type ryanodine receptor
Brain ryanodine receptor-calcium release channel
Brain-type ryanodine receptor
Type 3 ryanodine receptor
Skeletal muscle calcium release channel
Skeletal muscle ryanodine receptor
Skeletal muscle-type ryanodine receptor
Type 1 ryanodine receptor
Location in the cell
Cytoplasmic Reliability : 1.723 Nuclear Reliability : 2.296
Sequence
CDS
ATGGATGCCCTTGGCGGGGAGACGACATTCGCAGATGTCCAAGGCGATAATTTCGTGCCGGCTTGCACACTTGGGGTCGGGCAGAAGGCCAGGTTGACATACGGACAAGACGTGAACACTCTAAAATACTTCACGACCTGCGGTCTTCAAGAGGGATACGAACCTTTCTGTGTCAACATGAAAAGAGATGTCACTCATTGGTACACAAAAGACCAGCCCATCTATGAAAACACAGATGAAATGGCCGACACAAGAATCGATGTAACCAGGATACCAGCTGGATCAGAGACTCCACCGTGTCTGAAAATATCTCACAACACATTCGAAACGATGGAAAAAGCCAACTGGGAGTTTCTGCGTTTGTCTCTACCGGTCATTTGCCATTCGCAGTTTATAGATGAATCAGAGAAAGCACGACGCTGGGTAGAGATTAAGGAACGTCAGCAGATCCTCATGAAAGAGGCCACCGAAGCTCAGATACCAGCTCACATCGACCAAATCATGAGGAGTGGATTTACTATGAATGACATAAAAGGTCTGCACTACGAAGACAATCAAGAAGAGATCCAGAGCTCCAAAGTGAAACGTCAGCCGTCCAGACCACCACGACAACAAGTAAATGGAATTCATCGGTCCACCAGTGAAGCAGAAATGGCGAAATATGAATTAGGCGCTCAAACTTTAGCGCCAGATGAGAAGAAAGATAAAAGAGGACGTTCTCCTTTCAAATTTTTCAAGAGCAAACGTGCCGAGAGCAGTGATCGCGCCAAAATCCGCAAGTCCAAAACTCCAGATCCCTTCAGTGACACCGAACTGTCTCCTGACAGGGGGACGAAGAGACCCAACCCTCAGATCAAGGTGTCTCAGCCGAACCAAAGGTATAATAATGGCACGCAGCCCCGAGCAAGCAGACCCAACTTGTATGGAAGTCAAAGCGGTCTTAACATGGCGACTCCAACTCAGGAGAGAAAGCAGTTAATGACAACCACGAATCTGTCGGCCGCTGCGACGGAGACCGTCGGCAACGAGATATTCGACTCCGAGTGCTTAAAGCTCATTAATGAATACTTCTACGGGGTCAGAATATTCCCTGGCCAAGACCCAACTCACGTGTACATTGGGTGGGTGACCACTCAGTACCATCTGCACTCGAAAGACTTCAACCAAAACAAGGTGATGAAGTCATCAGTCATCATCACCGACGACTACGACCGGGTCATTGAAAGCGTAAACCGTCAGTCCTGCTACATGGTGCGAGCTGATGAGCTTTACAACGAAGTGATGGCGGAAGCTACGGCCAAGGGCGCGTCCCAAGGAATGTTTATTGGATGTTCAGTGGACACTTCCACCGGGACTGTAGCGTTCACATGCGAGGGAAAAAGCACTAGCATTAAATTCAAGATGGAGCCAGAGACCAAATTATTCCCGGCGATCTTCGTCGAAGCGACATCGAAAGAGATCCTCCAGATAGAGCTGGGCAGGTCTCCGACAAGTCTACCGCTAAGTGCCGCGGTGCTACCAACGAGCGACAAGCACGTCACGCCGCAGTTCCCGCCCAGGCTGAAAGTGCAGTGTCTCAAGCCGCATCAGTGGGCTAGAGTACCAAATTCATCGTTGCAAGTGCACGCTCTAAAGCTGTCTGACATACGAGGCTGGTCGATGCTGTGCGAAGACGCAGTTTCTATGCTCGCATTGCACATACCAGAGGAAGACCGGTGCATCGACATATTGGAGCTTATCGAAATGGATAAGCTGTTGAGCTTCCACTCGCACACCCTCACGTTATATGCAGCTTTATGCTACCAAAGCAACTACAGGGCTGCCCACGCTTTATGCCAGCATGTAGATCAAAAGCAATTGTTATACGCCATCAAGTCCCAGTACATGTCCGGGCCACTGCGCCAGGGTTTCTACGATCTACTCATTGCTCTACATTTAGAATCGCACGCAACCACGATGGAAACCTGCAAGAATGAATTTGTGATACCCCTGGGGCCGGAGCTGAAGGTTCTGTACGACGAGCCCGACATGGGGCACAGCCTCCGCTCGCTGCAGACGGAGAGCGTCAGACCGCAGTTGGAAATGACCGATATCACGGAAAAAAGTATAATGGACATAAGTAATCTGTACTCTCCGAAGTTCCCTCTGGAAGTTGTCCGTGAATTCGTGATGCAGGCACTCGCGGAGGCCGTGGAAACTAACCAGGTCCACAACAGAGATCCGGTCGGTGGAAGCAACGAGAATCTCTTCCTGCCGCTAATTAAGCTCGTGGACCGTCTACTCCTGGTCGGAAAAATGCGCGACGAAGACGTTGAGAAACTTCTCATCATGATCAACCCCGAGACCTGGGATCCAACGTTCAATAAAGAAGGCAAAGACGAACACCGAAAAGGACTTCTTCACATGAAAATGGCTGAAGGCGCTAAACTGCAAATGTGTTATTTGCTCCAACATCTGAACGACATGCAGCTACGTCACAGGGTTGAATCTATAGTGGCTTTTGCACATGACTTTGTCGGGGACCTGCAGTCCGACCAGTTGAGACGCTACACTGAGATCAAGCAGTCAGATTTACCGAGTGCGGTTGCAGCCAAGAAGACCAGAGAATTCCGTTGTCCTCCTAGGGAACAGATGAACGCAATCCTGAGTTTCAAACATCTTGAAGATATCGATAAGGAGAACTGTCCTTGTGGCGAAGAACTCATCGCTAGGATGAACGGCTTCCACGAAAGTCTCATGGTCCACGTATCTCTAAACGCCCTGCAAGAACCTGATCCTGAGGAGCCCACTGAACCAGAAATAAAGCCGGGGGCCATTAATAAATTATATAGCCTTATAAATACCGTCAAGGAACTAGAAGAGGAACCCAAGACATTAGAGGAACCAGCCAAGAAGACTCCGGAGGAACGTTTCCGGAAAGTACTCATACAGACGATCGTGAATTGGGCGGAGGAATCGCAGATTGAGACTCCCAAGTTAGTCAGGGAGATGTTTAGCCTACTGGTTCGCCAGTATGACGCCGTAGGAGAACTGATCCGAGCTCTCGAAAAGACTTACGTCATCAATGCGAAGACCAAGCAGGACGTGGCCGAGATGTGGGTGGGACTCAGTCAGATCAGAGCCCTGCTCCCGGTGCAAATGAGTCAAGAAGAGGAGGAACTCATGAGGAAAAGGCTTTGGAAACTGGTCAACAACCACACTTTCTTCCAGCATCCAGATCTGATAAGAGTATTGAGGGTACACGAAAACGTGATGGCTGTAATGATGAATACTTTGGGAAGGAGAGCCCAAGCGCAGTCTGATGCTGCACCGAACCAGCCGCTTGCCGAGGATACCAAGGAGAAGGACACTTCGCATGAAATGGTTGTGGCCTGTTGTCGATTCCTCTGCTACTTCTGTCGCACCGGTCGTCAGAATCAAAAAGCCATGTTCGATCACTTTGACTTCTTGCTGGAGAACTCCAATATTCTACTGTCGAGACCTTCGTTGCGAGGTTCGACTCCTTTGGATGTTGCCTACTCCAGTTTGATGGAGAACACGGAATTGGCTTTAGCTTTGAGGGAGCACTATCTTGAGAAAATAGCGGTATACCTTTCTCGTTGTGGACTACAGAGCAACTCTGAGTTAGTGGAAAAGGGGTATCCAGACCTTGGATGGGATCCGGTAGAAGGCGAGAGATATTTGGACTTCCTGAGATTTTGTGTTTGGGTAAATGGTGAAAGCGTAGAAGAAAACGCCAACTTAGTGATCCGCTTGCTAATCAGGCGGCCGGAGTGTCTTGGCCCGGCCCTTCGGGGAGAAGGCGAAGGTCTACTGAAGGCTATTGTGGACGCGAACAAAATGAGCGAAAGGATCGCAGATAGAAGAAAACTAAGAGAGATAGAACAGGAAGGGGATATTAATTTCAGCCATCCACTCCCGGAGTCGGACGACGACGAGGATTACATCGATACTGGAGCGGCCATACTTAACTTCTACTGCACCCTGGTCGATCTGCTGGGGCGCTGTGCTCCGGATGCGGCAGTAATTGCTCTTGGTAAGAACGAGTCGCTTCGGGCCCGTGCCATTCTGCGCTCCTTGGTACCGTTGGAAGATCTTCAAGGAGTGCTCAGTCTTCGGTTCACCCTCAACAATCCCGCTGCCGGTGAAGAGAGACCCAAGTCCGACATGCCATCCGGACTAATCCCGGGACACAAGCAGAGCGTCGGACTTTTCTTAGAACGAGTCTATGGCATCGAGACCCAAGAGCTTTTCTACAAGCTTCTCGAAGAAGCTTTCTTGCCAGACTTGAGAGCTGCCACTATGCTAGACAGGAACGACGGCTGCGAATCGGACATGGCGCTTTCAATGAACCGGTACATCGGGAATTCGATCCTCCCGCTGCTGATCAAACACGCCAACTTCTACAACGAAGCCGAGAACTACGCCAGTCTGCTGGACGCCACGCTGCACACCGTCTACAGATTATCCAAAAACCGCATGCTGACAAAAGGCCAACGTGAGGCTGTCTCCGACTTCTTGGTGGCTCTGACGTCAGCGATGCAGCCCTCCATGCTGTTGAAGCTGCTGAGGAAGCTCACGGTTGACGTCTCGAGACTGTCCGAGTACACCACCGTGGCTCTCAGACTCCTGACGCTGCACTACGAACGTTGCGCCAAGTACTACGGCAGCACTGGTGGGCAGGGAATATACGGAGCATCTTCTGATGAGGAGAAGCGTCTCACCATGATGCTCTTCTCTAACATCTTCGATTCCCTCAGCAAGATGGACTACGAACCCGAGCTGTTTGGGAAAGCTTTACCCTGTCTCATCGCTATAGGCTGTGCTCTACCACCAGACTACTCTCTATCGAAGAATTACGACGATGAATTCTATGGAAAGGAGCAAGCATCAGTAGGCTCCGACAACCCGCAGTACGATCCCCAGCCGATCAACACCTCATCAGTCGCCCTGAACAACGACTTGAACACGATCGTGCAGAAGTTCTCCGAGCATTACCACGACGCCTGGGCCTCCAGGAAAATTGAGAACGGCTGGGTGTACGGCGAGTCTTGGTCAGACAGCCAGAAGACGCATCCACGACTGAAGCCTTACAACATGCTCAATGACTATGAGAAAGAGCGGTACAAAGAACCGGTCCGCGAGTCGCTGAAGGCACTTCTGGCTCTGGGCTGGTCCGTGGAACATTCCGACGTAGACCTGCCCGCCAACAGTCGCGGCTCTGTGCGAAGACAGTCCAAGTCCGGACTGACCGACTCAGCAACCCCTTTCAACTACAACCCTCACCCCGTGGACATGACGAATCTGACGCTGTCCAGAGAAATGCAGAACATGGCCGAGAGACTGGCTGACAACGCACATGACATCTGGGCTAAGAAGAAAAAAGAAGAACTAGTTACTAATGGAGGGGGAATCCATCCTCAACTAGTTCCTTACGACCTCCTAACTGATAAAGAAAAGAAGAAAGACCGAGAACGCTCACAGGAGTTCCTCAAGTACCTCCAATATCAGGGCTATAAGCTGCACAGGCCAAGCAAAATCCCGCAGAGCGACACGGAACAGACCTCTGCCGGAGTGGCAATAGAGCTGAGATTCGCTTACTCGTTGCTAGAAAAGTTGATCCAGTACATAGACAGGGCTACAATCAACATGAAACTCCTGAAGCCATCGACGACGTTCAGTCGTAGAAGCAGCTTCAAGACAAGCACCAGAGATATAAAGTTCTTCTCGAAAGTGGTTCTCCCTCTAATGGAGAAGTACTTCTCGACCCATCGCAACTACTTCATAGCGGTTGCAACTGCCACCAATAATGTGGGAGCCGCGAGCCTTAAAGAGAAAGAAATGGTCGCCGCTTTGTTCTGTAAGCTGGCCAGCCTGCTTCGATCTAGACTAGCAGCTTTTGGCCCCGACGTCCGCATCACCGTTCGCTGTCTCCAAGTCCTCGTCAAGGGCATTGACGCTAAATCTCTGGTCAAAAACTGCCCGGAATTCATTCGGACTTCAATGCTGACCTTTTTCAACAACATGGCCGACGATGTTGGGCACACCATCATTAATTTGCAGGATGGCAAGTACGCGCACCTCCGCGGTACCCATTTGAAAACGTCTACATCTCTTGGATACATTAACGGAGTACAATTACCTGTACTGACTGCTATGTTCGATCACCTAGCGAACTGCGAATACGGATCAGATCTGCTTTTGGACGAAATTCAAGTTGCGTCATACAAGATGCTCGGGTCACTCTACACGTTGGGCACTGATGTAACGCTGACCCACGACCGCAAGTACTTGAAGACGGAGATTGAAAGACACAAGCCTGCTTTGGGTTCGTGTCTGGGCGCTTTCAGTTCAACCTTCCCCGTGGCGTATCTGGAGCCGCATCTCAACAAACACAACCAGTTTTCTCTGCTTAACAGGATCGCTGAGCATTCGCTCGAGGCCCAAGATATAATGGCGAAAATGGAGCAATCTATGCCGACTCTAGAGACGATCTTGAACGAAGTGGACCAATTCGTTGAATCAGACAAGACGTATAATGAAGCGCCGCATATTATCGACGTGGTCCTGCCATTACTGTGCTCTTACCTGCCGTTCTGGTGGGCGCAGGGACCCGACAACGTCACCCCTACTGGAGGGAACCACGTTACCATGGTAACGGCGGAGCACATGAACCAACTCCTCAAGAACGTGCTCAAACTAATCAAAAAGAACATCGGCAATGAAAGCGCCCCGTGGATGACGAGGATCGCCACTTACACCCAGCAGATCATCATCAACAGCTCCGAAGAGTTGCTCAGGGATTCCTTCCTGCCTCTCGCCGAGAGAGTCAGGAAACGAACAGACAATATGTTCCACAAGGAAGAGAGCTTGAGGGGCTTCATAAAGTCCTCGACCGACGACACTTCGCAAGTAGAGTCTCAGATACAAGAAGACTGGCAGTTACTGGTGCGAGACATCTACTCCTTCTATCCGCTTCTCATCAAGTACGTGGACTTGCAGAGGAATCACTGGTTGAGGAATAATGTTCCAGAGGCCGAGGAGCTATACAACCACGTGGCCGAAATCTTCAACATCTGGTCGAAGAGTCAATACTTCTTGAAGGAAGAACAAAACTTTATATCGGCAAACGAAATCGATAATATGGTCTTGATAATGCCAACAGCCACACGCCGAGTCACGGCCGTGGTCGACGGCACGTCGCAAAGTGGTGGGAAGAAAAAGAAGAAGCATCGCGACAAGAAACGTGACAAGGACAAGGAAGTTCAGGCGTCTCTGATGGTCGCCTGTCTGAAGCGGTTGCTGCCGGTAGGACTGAATCTCTTCGCTGGAAGGGAACAGGAACTGGTACAGCACTGCAAGGATAGGTTCCTGAAGAAAATGTCCGAGCATGACGTTGCCGAATTCGCGAAGACCCAGCTGACGCTACCGGATAAAATAGACCCGGCCGACGAGATGTCGTGGCAACACTACCTCTACAGCAAATTAGGGTCGAAAAGCAAGACTGCCATCACTCTGGAGAATGCCGAGAACAAAGCGAAAATCATCGACGACACCGTTGAGAGGATCGTCGCGATGAGCAAAGTGCTCTTCGGATTGCATATGATCGACCATCCGCAACAAATGAGCAAGAACGTTTATCGATCAGTGGTCTCCATACAAAGGAAGAGGGCCGTGATAGCGTGTTTCAGACAGACGTCCCTACATTCGCTGCCGAGACATCGTGCTTGTAACATCTTCGCGAGGACTTATTACGAGCTGTGGCTGGAGGAAGAAAACATCGGACAAGAAGTCATGATTGAGGATTTGACGCAATCGTTCGAGGATGCTGAACTCAAGAAGAGTGATGTGGTAGAGGAGGATGGGAAGCCAGATCCACTGACCCAGTTGGTCACGACCTTCTGCAGGGGAGCTATGACGGAACGGTCCGGAGCCTTGCAGGAAGATCCACTGTACATGTCGTACGCGCATATCATTGCGAAGTCATGCGGAGAAGAGGAGGAGGAAGGAGGCGGAGAGGAGGAGGAGGGAGGAGGAGAAGCGGAAGGGGAAGAGGAAGGTCGGGCCAGTATACACGAGCAAGAAATGGAGAAGCAGAAGCTACTCTTCCACCAGGCGAGGCTCGCCAACAGGGGGGTCGCGGAGATGGTGCTCCTACACATATCTGCGTCCAAGGGGGTGCCCAGTGAAATGGTCATGAAAACGCTACAGCTCGGCATATCCATACTGCGCGGCGGGAACATTGACATACAGATGGGAATGCTGAACCATTTGAAAGACAAGAAAGACGTGGGCTTCTTCACGTCCATAGCTGGCCTCATGAACTCTTGCTCTGTGTTGGATCTGGATGCCTTCGAAAGGAACACTAAGGCTGAAGGTCTCGGGGTGGGGCTGGAGGGAGCCGCCGGCGAAAAGAACATGCACGACGCAGAGTTCACGTGCGCTCTCTTCAGATTCATCCAGCTGACTTGCGAAGGGCACAACTTGGATTGGCAGAACTACCTCCGGACCCAGGCCGGGAACACGACTACCGTGAACGTCGTGATCTGCACTGTGGACTACCTGCTACGTCTCCAGGAGTCCATCATGGACTTCTACTGGCACTATTCGAGCAAGGAACTGATCGACCCGGCCGGTAAAGCGAACTTCTTTAAGGCGATCGGCGTCGCTTCGCAAGTCTTCAACACGCTCACTGAAGTCATCCAGGGACCTTGTACTCAGAACCAGCAGGCCTTAGCCCACTCCAGACTGTGGGACGCTGTGGGAGGGTTTCTCTTCCTGTTCTCGCACATGCAGGACAAGCTGTCGAAGCACTCGTCACAAGTGGACCTGCTGAAGGAGCTACTCAATTTGCAGAAGGACATGATCACCATGATGCTTTCGATGCTCGAAGGAAACGTCGTCAATGGTACCATCGGTAAGCAGATGGTGGACACATTAGTAGAGTCGGCTTCAAACGTGGAACTGATCCTCAAGTACTTCGACATGTTCCTGAAGCTGAAGGACTTGACCTCTAGCGCCAGTTTCCAAGAAATCGACGCCAATAACGACGGCTGGGTCCTGCCGAAAGACTTCAAAGAGAAGATGGAACAGCAAAAGAGTTACACGCCCGAAGAGATAGAGTTCTTACTCGCTTGCTGCGAGACGAACCACGACGGTAAACTTGACTACGTGGGCTTCTGCGACCGTTTCCACGAACCGGCCAAAGAAATCGGCTTCAACCTTGCCGTACTGCTGACCAACCTCTCGGAGCACATGCCCAACGAACCAAGATTGGCCCGTTTCCTGGAAACAGCTGGCTCGGTGCTGAACTACTTCGAGCCATTCCTCGGCCGCATCGAGATCATGGGTGGATCGAAGCGCATCGAACGAGTCTACTTTGAGATCAAAGAATCTAATATTGAACAGTGGGAGAAGCCGCAGATCAAGGAATCAAAGCGCGCGTTTTTCTACAGCATCGTAACCGAAGGCGGCGACAAGGAGAAGCTGGAAGCGTTCGTGAACTTCTGCGAGGACGCCATCTTTGAGATGACCCACGCGTCGGGTCTGATGGCCGCTTCGGACGACACCGCCGGCGGACCTAAGAACAGGGAGGCCAGCTACATGTACATGGGAGACGACGATGATGATCGTGCCGGCAAGGATCCGTTCCGTCGCGGCATCCAATCAGTGAAAGACGGCATCTCCACGGCGTTCTCGTCTTTATCACCGTCGAACATAAAGGCGAAAATTGCAGATCTACAGCAAATGCCACCGGCAGAATTGGCCGTCGGCTTCTTCAAAATGTTCTTCTATATGTTCTATTACTTGGGCTATGGAGTGCTGGTCGTTGTCAGGTACATATTCGGAGTGCTCCTTGGACTAATGCGGGGCCCGCAGGTGGAGGAGCCACCACCAGAGCCAACTGAAGAGGAGAAAATCGGTCCGAGACATCTGCCAGCTTTACCGCCTGCTGATGACACTGGACAGATGCAAGTCTCGGCCTTCGGTTTGGACATAACTAAAGAAGACAACGGGCAGATCCAGGTGAAGCCGCACGAGTCTCCTACTACATCGACGCCGTCTTCAGGTGAAGAGGCTGACGCGTCTCTCGACGAGGGACTGGAACACTCCGAAGAGCAACGACCACCGTCGCTGATCGACTTATTAGGCGGGGAACAAGCAAAGAAACAAGCTCTAGAGCGCATTGAGGCTCAGGCCGCGCAACAGGCCGCCATGTCGGCGATCGAGGCCGAGAGCAAGAAGGCCGTCCAGGGTCCTGCTTCGTCGTCAGCTCTCTCCCAAGTGGATCTGTCGCAGTACACACGGCGAGCCGTTTCGTTTTTGGCCAGAAACTTCTACAACTTGAAATACGTGGCCCTCGTGCTGGCTTTTTGCATCAACTTCGTGCTTTTGTTTTATAAGGTTTCGACTCTTGATGGCGAAAAAGCCGAAGGTTCGGGCATCGGAGATATCATTGCGGGGTCTGGTTCTGGGCAAGGCTCTGGCAGTGGTGACGTTACATGGTATAGGCCTAAAGTCACCAAATTCAGCGGAGAATGGGCAGCAGCGCCCTCCGAGGATTCCATACTGTCCTTCATATTTCTGCAGCAAAGCATGCATGAGTTGGAAGGAAGGGGCCGGATTTTATGTAAAACAATTTAG
Protein
MDALGGETTFADVQGDNFVPACTLGVGQKARLTYGQDVNTLKYFTTCGLQEGYEPFCVNMKRDVTHWYTKDQPIYENTDEMADTRIDVTRIPAGSETPPCLKISHNTFETMEKANWEFLRLSLPVICHSQFIDESEKARRWVEIKERQQILMKEATEAQIPAHIDQIMRSGFTMNDIKGLHYEDNQEEIQSSKVKRQPSRPPRQQVNGIHRSTSEAEMAKYELGAQTLAPDEKKDKRGRSPFKFFKSKRAESSDRAKIRKSKTPDPFSDTELSPDRGTKRPNPQIKVSQPNQRYNNGTQPRASRPNLYGSQSGLNMATPTQERKQLMTTTNLSAAATETVGNEIFDSECLKLINEYFYGVRIFPGQDPTHVYIGWVTTQYHLHSKDFNQNKVMKSSVIITDDYDRVIESVNRQSCYMVRADELYNEVMAEATAKGASQGMFIGCSVDTSTGTVAFTCEGKSTSIKFKMEPETKLFPAIFVEATSKEILQIELGRSPTSLPLSAAVLPTSDKHVTPQFPPRLKVQCLKPHQWARVPNSSLQVHALKLSDIRGWSMLCEDAVSMLALHIPEEDRCIDILELIEMDKLLSFHSHTLTLYAALCYQSNYRAAHALCQHVDQKQLLYAIKSQYMSGPLRQGFYDLLIALHLESHATTMETCKNEFVIPLGPELKVLYDEPDMGHSLRSLQTESVRPQLEMTDITEKSIMDISNLYSPKFPLEVVREFVMQALAEAVETNQVHNRDPVGGSNENLFLPLIKLVDRLLLVGKMRDEDVEKLLIMINPETWDPTFNKEGKDEHRKGLLHMKMAEGAKLQMCYLLQHLNDMQLRHRVESIVAFAHDFVGDLQSDQLRRYTEIKQSDLPSAVAAKKTREFRCPPREQMNAILSFKHLEDIDKENCPCGEELIARMNGFHESLMVHVSLNALQEPDPEEPTEPEIKPGAINKLYSLINTVKELEEEPKTLEEPAKKTPEERFRKVLIQTIVNWAEESQIETPKLVREMFSLLVRQYDAVGELIRALEKTYVINAKTKQDVAEMWVGLSQIRALLPVQMSQEEEELMRKRLWKLVNNHTFFQHPDLIRVLRVHENVMAVMMNTLGRRAQAQSDAAPNQPLAEDTKEKDTSHEMVVACCRFLCYFCRTGRQNQKAMFDHFDFLLENSNILLSRPSLRGSTPLDVAYSSLMENTELALALREHYLEKIAVYLSRCGLQSNSELVEKGYPDLGWDPVEGERYLDFLRFCVWVNGESVEENANLVIRLLIRRPECLGPALRGEGEGLLKAIVDANKMSERIADRRKLREIEQEGDINFSHPLPESDDDEDYIDTGAAILNFYCTLVDLLGRCAPDAAVIALGKNESLRARAILRSLVPLEDLQGVLSLRFTLNNPAAGEERPKSDMPSGLIPGHKQSVGLFLERVYGIETQELFYKLLEEAFLPDLRAATMLDRNDGCESDMALSMNRYIGNSILPLLIKHANFYNEAENYASLLDATLHTVYRLSKNRMLTKGQREAVSDFLVALTSAMQPSMLLKLLRKLTVDVSRLSEYTTVALRLLTLHYERCAKYYGSTGGQGIYGASSDEEKRLTMMLFSNIFDSLSKMDYEPELFGKALPCLIAIGCALPPDYSLSKNYDDEFYGKEQASVGSDNPQYDPQPINTSSVALNNDLNTIVQKFSEHYHDAWASRKIENGWVYGESWSDSQKTHPRLKPYNMLNDYEKERYKEPVRESLKALLALGWSVEHSDVDLPANSRGSVRRQSKSGLTDSATPFNYNPHPVDMTNLTLSREMQNMAERLADNAHDIWAKKKKEELVTNGGGIHPQLVPYDLLTDKEKKKDRERSQEFLKYLQYQGYKLHRPSKIPQSDTEQTSAGVAIELRFAYSLLEKLIQYIDRATINMKLLKPSTTFSRRSSFKTSTRDIKFFSKVVLPLMEKYFSTHRNYFIAVATATNNVGAASLKEKEMVAALFCKLASLLRSRLAAFGPDVRITVRCLQVLVKGIDAKSLVKNCPEFIRTSMLTFFNNMADDVGHTIINLQDGKYAHLRGTHLKTSTSLGYINGVQLPVLTAMFDHLANCEYGSDLLLDEIQVASYKMLGSLYTLGTDVTLTHDRKYLKTEIERHKPALGSCLGAFSSTFPVAYLEPHLNKHNQFSLLNRIAEHSLEAQDIMAKMEQSMPTLETILNEVDQFVESDKTYNEAPHIIDVVLPLLCSYLPFWWAQGPDNVTPTGGNHVTMVTAEHMNQLLKNVLKLIKKNIGNESAPWMTRIATYTQQIIINSSEELLRDSFLPLAERVRKRTDNMFHKEESLRGFIKSSTDDTSQVESQIQEDWQLLVRDIYSFYPLLIKYVDLQRNHWLRNNVPEAEELYNHVAEIFNIWSKSQYFLKEEQNFISANEIDNMVLIMPTATRRVTAVVDGTSQSGGKKKKKHRDKKRDKDKEVQASLMVACLKRLLPVGLNLFAGREQELVQHCKDRFLKKMSEHDVAEFAKTQLTLPDKIDPADEMSWQHYLYSKLGSKSKTAITLENAENKAKIIDDTVERIVAMSKVLFGLHMIDHPQQMSKNVYRSVVSIQRKRAVIACFRQTSLHSLPRHRACNIFARTYYELWLEEENIGQEVMIEDLTQSFEDAELKKSDVVEEDGKPDPLTQLVTTFCRGAMTERSGALQEDPLYMSYAHIIAKSCGEEEEEGGGEEEEGGGEAEGEEEGRASIHEQEMEKQKLLFHQARLANRGVAEMVLLHISASKGVPSEMVMKTLQLGISILRGGNIDIQMGMLNHLKDKKDVGFFTSIAGLMNSCSVLDLDAFERNTKAEGLGVGLEGAAGEKNMHDAEFTCALFRFIQLTCEGHNLDWQNYLRTQAGNTTTVNVVICTVDYLLRLQESIMDFYWHYSSKELIDPAGKANFFKAIGVASQVFNTLTEVIQGPCTQNQQALAHSRLWDAVGGFLFLFSHMQDKLSKHSSQVDLLKELLNLQKDMITMMLSMLEGNVVNGTIGKQMVDTLVESASNVELILKYFDMFLKLKDLTSSASFQEIDANNDGWVLPKDFKEKMEQQKSYTPEEIEFLLACCETNHDGKLDYVGFCDRFHEPAKEIGFNLAVLLTNLSEHMPNEPRLARFLETAGSVLNYFEPFLGRIEIMGGSKRIERVYFEIKESNIEQWEKPQIKESKRAFFYSIVTEGGDKEKLEAFVNFCEDAIFEMTHASGLMAASDDTAGGPKNREASYMYMGDDDDDRAGKDPFRRGIQSVKDGISTAFSSLSPSNIKAKIADLQQMPPAELAVGFFKMFFYMFYYLGYGVLVVVRYIFGVLLGLMRGPQVEEPPPEPTEEEKIGPRHLPALPPADDTGQMQVSAFGLDITKEDNGQIQVKPHESPTTSTPSSGEEADASLDEGLEHSEEQRPPSLIDLLGGEQAKKQALERIEAQAAQQAAMSAIEAESKKAVQGPASSSALSQVDLSQYTRRAVSFLARNFYNLKYVALVLAFCINFVLLFYKVSTLDGEKAEGSGIGDIIAGSGSGQGSGSGDVTWYRPKVTKFSGEWAAAPSEDSILSFIFLQQSMHELEGRGRILCKTI
Summary
Description
Intracellular calcium channel that is required for proper muscle function during embryonic development and may be essential for excitation-contraction coupling in larval body wall muscles.
Calcium channel that mediates the release of Ca(2+) from the sarcoplasmic reticulum into the cytoplasm and thereby plays a key role in triggering cardiac muscle contraction. Aberrant channel activation can lead to cardiac arrhythmia. In cardiac myocytes, calcium release is triggered by increased Ca(2+) levels due to activation of the L-type calcium channel CACNA1C. The calcium channel activity is modulated by formation of heterotetramers with RYR3. Required for cellular calcium ion homeostasis. Required for embryonic heart development (By similarity).
Calcium channel that mediates the release of Ca(2+) from the sarcoplasmic reticulum into the cytoplasm and thereby plays a key role in triggering cardiac muscle contraction. Aberrant channel activation can lead to cardiac arrhythmia. In cardiac myocytes, calcium release is triggered by increased Ca(2+) levels due to activation of the L-type calcium channel CACNA1C. The calcium channel activity is modulated by formation of heterotetramers with RYR3. Required for cellular calcium ion homeostasis. Required for embryonic heart development.
Calcium channel that mediates the release of Ca(2+) from the sarcoplasmic reticulum into the cytoplasm and thereby plays a key role in triggering cardiac muscle contraction. Aberrant channel activation can lead to cardiac arrhythmia. In cardiac myocytes, calcium release is triggered by increased Ca(2+) levels due to activation of the L-type calcium channel CACNA1C. Required for cellular calcium ion homeostasis. Required for embryonic heart development (By similarity). The calcium channel activity is modulated by formation of heterotetramers with RYR3.
Calcium channel that mediates the release of Ca(2+) from the sarcoplasmic reticulum into the cytoplasm in muscle and thereby plays a role in triggering muscle contraction. May regulate Ca(2+) release by other calcium channels. Calcium channel that mediates Ca(2+)-induced Ca(2+) release from the endoplasmic reticulum in non-muscle cells. Contributes to cellular calcium ion homeostasis (By similarity). Plays a role in cellular calcium signaling.
Calcium channel that mediates the release of Ca(2+) from the sarcoplasmic reticulum into the cytoplasm in muscle and thereby plays a role in triggering muscle contraction. May regulate Ca(2+) release by other calcium channels. Calcium channel that mediates Ca(2+)-induced Ca(2+) release from the endoplasmic reticulum in non-muscle cells. Plays a role in cellular calcium signaling. Contributes to cellular calcium ion homeostasis. Isoform 2 lacks a predicted transmembrane segment and does not form functional calcium channels by itself; however, it can form tetramers with isoforms that contain the full complement of transmembrane segments and modulate their activity.
Calcium channel that mediates the release of Ca(2+) from the sarcoplasmic reticulum into the cytoplasm and thereby plays a key role in triggering muscle contraction following depolarization of T-tubules (PubMed:3722165, PubMed:10388749, PubMed:10097181, PubMed:12732639, PubMed:22036948, PubMed:26245150, PubMed:27662087). Repeated very high-level exercise increases the open probability of the channel and leads to Ca(2+) leaking into the cytoplasm (By similarity). Can also mediate the release of Ca(2+) from intracellular stores in neurons, and may thereby promote prolonged Ca(2+) signaling in the brain. Required for normal embryonic development of muscle fibers and skeletal muscle. Required for normal heart morphogenesis, skin development and ossification during embryogenesis (By similarity).
Calcium channel that mediates the release of Ca(2+) from the sarcoplasmic reticulum into the cytoplasm and thereby plays a key role in triggering muscle contraction following depolarization of T-tubules (PubMed:18003898, PubMed:7515481, PubMed:7621815, PubMed:21156754). Repeated very high-level exercise increases the open probability of the channel and leads to Ca(2+) leaking into the cytoplasm (PubMed:18268335). Can also mediate the release of Ca(2+) from intracellular stores in neurons, and may thereby promote prolonged Ca(2+) signaling in the brain (PubMed:22036948). Required for normal embryonic development of muscle fibers and skeletal muscle (PubMed:7515481). Required for normal heart morphogenesis, skin development and ossification during embryogenesis (PubMed:18003898, PubMed:7515481).
Calcium channel that mediates the release of Ca(2+) from the sarcoplasmic reticulum into the cytoplasm and thereby plays a key role in triggering muscle contraction following depolarization of T-tubules (PubMed:11316255). Repeated very high-level exercise increases the open probability of the channel and leads to Ca(2+) leaking into the cytoplasm (By similarity). Can also mediate the release of Ca(2+) from intracellular stores in neurons, and may thereby promote prolonged Ca(2+) signaling in the brain. Required for normal embryonic development of muscle fibers and skeletal muscle. Required for normal heart morphogenesis, skin development and ossification during embryogenesis (By similarity).
Calcium channel that mediates the release of Ca(2+) from the sarcoplasmic reticulum into the cytoplasm and thereby plays a key role in triggering muscle contraction following depolarization of T-tubules (By similarity). Repeated very high-level exercise increases the open probability of the channel and leads to Ca(2+) leaking into the cytoplasm (By similarity). Can also mediate the release of Ca(2+) from intracellular stores in neurons, and may thereby promote prolonged Ca(2+) signaling in the brain. Required for normal embryonic development of muscle fibers and skeletal muscle. Required for normal heart morphogenesis, skin development and ossification during embryogenesis (By similarity).
Calcium channel that mediates the release of Ca(2+) from the sarcoplasmic reticulum into the cytoplasm and thereby plays a key role in triggering muscle contraction following depolarization of T-tubules (PubMed:11741831, PubMed:16163667). Repeated very high-level exercise increases the open probability of the channel and leads to Ca(2+) leaking into the cytoplasm (PubMed:18268335). Can also mediate the release of Ca(2+) from intracellular stores in neurons, and may thereby promote prolonged Ca(2+) signaling in the brain. Required for normal embryonic development of muscle fibers and skeletal muscle. Required for normal heart morphogenesis, skin development and ossification during embryogenesis (By similarity).
Calcium channel that mediates the release of Ca(2+) from the sarcoplasmic reticulum into the cytoplasm and thereby plays a key role in triggering cardiac muscle contraction. Aberrant channel activation can lead to cardiac arrhythmia. In cardiac myocytes, calcium release is triggered by increased Ca(2+) levels due to activation of the L-type calcium channel CACNA1C. The calcium channel activity is modulated by formation of heterotetramers with RYR3. Required for cellular calcium ion homeostasis. Required for embryonic heart development (By similarity).
Calcium channel that mediates the release of Ca(2+) from the sarcoplasmic reticulum into the cytoplasm and thereby plays a key role in triggering cardiac muscle contraction. Aberrant channel activation can lead to cardiac arrhythmia. In cardiac myocytes, calcium release is triggered by increased Ca(2+) levels due to activation of the L-type calcium channel CACNA1C. The calcium channel activity is modulated by formation of heterotetramers with RYR3. Required for cellular calcium ion homeostasis. Required for embryonic heart development.
Calcium channel that mediates the release of Ca(2+) from the sarcoplasmic reticulum into the cytoplasm and thereby plays a key role in triggering cardiac muscle contraction. Aberrant channel activation can lead to cardiac arrhythmia. In cardiac myocytes, calcium release is triggered by increased Ca(2+) levels due to activation of the L-type calcium channel CACNA1C. Required for cellular calcium ion homeostasis. Required for embryonic heart development (By similarity). The calcium channel activity is modulated by formation of heterotetramers with RYR3.
Calcium channel that mediates the release of Ca(2+) from the sarcoplasmic reticulum into the cytoplasm in muscle and thereby plays a role in triggering muscle contraction. May regulate Ca(2+) release by other calcium channels. Calcium channel that mediates Ca(2+)-induced Ca(2+) release from the endoplasmic reticulum in non-muscle cells. Contributes to cellular calcium ion homeostasis (By similarity). Plays a role in cellular calcium signaling.
Calcium channel that mediates the release of Ca(2+) from the sarcoplasmic reticulum into the cytoplasm in muscle and thereby plays a role in triggering muscle contraction. May regulate Ca(2+) release by other calcium channels. Calcium channel that mediates Ca(2+)-induced Ca(2+) release from the endoplasmic reticulum in non-muscle cells. Plays a role in cellular calcium signaling. Contributes to cellular calcium ion homeostasis. Isoform 2 lacks a predicted transmembrane segment and does not form functional calcium channels by itself; however, it can form tetramers with isoforms that contain the full complement of transmembrane segments and modulate their activity.
Calcium channel that mediates the release of Ca(2+) from the sarcoplasmic reticulum into the cytoplasm and thereby plays a key role in triggering muscle contraction following depolarization of T-tubules (PubMed:3722165, PubMed:10388749, PubMed:10097181, PubMed:12732639, PubMed:22036948, PubMed:26245150, PubMed:27662087). Repeated very high-level exercise increases the open probability of the channel and leads to Ca(2+) leaking into the cytoplasm (By similarity). Can also mediate the release of Ca(2+) from intracellular stores in neurons, and may thereby promote prolonged Ca(2+) signaling in the brain. Required for normal embryonic development of muscle fibers and skeletal muscle. Required for normal heart morphogenesis, skin development and ossification during embryogenesis (By similarity).
Calcium channel that mediates the release of Ca(2+) from the sarcoplasmic reticulum into the cytoplasm and thereby plays a key role in triggering muscle contraction following depolarization of T-tubules (PubMed:18003898, PubMed:7515481, PubMed:7621815, PubMed:21156754). Repeated very high-level exercise increases the open probability of the channel and leads to Ca(2+) leaking into the cytoplasm (PubMed:18268335). Can also mediate the release of Ca(2+) from intracellular stores in neurons, and may thereby promote prolonged Ca(2+) signaling in the brain (PubMed:22036948). Required for normal embryonic development of muscle fibers and skeletal muscle (PubMed:7515481). Required for normal heart morphogenesis, skin development and ossification during embryogenesis (PubMed:18003898, PubMed:7515481).
Calcium channel that mediates the release of Ca(2+) from the sarcoplasmic reticulum into the cytoplasm and thereby plays a key role in triggering muscle contraction following depolarization of T-tubules (PubMed:11316255). Repeated very high-level exercise increases the open probability of the channel and leads to Ca(2+) leaking into the cytoplasm (By similarity). Can also mediate the release of Ca(2+) from intracellular stores in neurons, and may thereby promote prolonged Ca(2+) signaling in the brain. Required for normal embryonic development of muscle fibers and skeletal muscle. Required for normal heart morphogenesis, skin development and ossification during embryogenesis (By similarity).
Calcium channel that mediates the release of Ca(2+) from the sarcoplasmic reticulum into the cytoplasm and thereby plays a key role in triggering muscle contraction following depolarization of T-tubules (By similarity). Repeated very high-level exercise increases the open probability of the channel and leads to Ca(2+) leaking into the cytoplasm (By similarity). Can also mediate the release of Ca(2+) from intracellular stores in neurons, and may thereby promote prolonged Ca(2+) signaling in the brain. Required for normal embryonic development of muscle fibers and skeletal muscle. Required for normal heart morphogenesis, skin development and ossification during embryogenesis (By similarity).
Calcium channel that mediates the release of Ca(2+) from the sarcoplasmic reticulum into the cytoplasm and thereby plays a key role in triggering muscle contraction following depolarization of T-tubules (PubMed:11741831, PubMed:16163667). Repeated very high-level exercise increases the open probability of the channel and leads to Ca(2+) leaking into the cytoplasm (PubMed:18268335). Can also mediate the release of Ca(2+) from intracellular stores in neurons, and may thereby promote prolonged Ca(2+) signaling in the brain. Required for normal embryonic development of muscle fibers and skeletal muscle. Required for normal heart morphogenesis, skin development and ossification during embryogenesis (By similarity).
Subunit
Homotetramer.
Homotetramer. Can also form heterotetramers with RYR3. Identified in a complex composed of RYR2, FKBP1B, PKA catalytic subunit, PRKAR2A, AKAP6, and the protein phosphatases PP2A and PP1. Interacts directly with FKBP1B, PKA, PP1 and PP2A (By similarity). Interacts with FKBP1A and FKBP1B; these interactions may stabilize the channel in its closed state and prevent Ca(2+) leaks. Interacts with CALM and S100A1; these interactions regulate channel activity. Interacts with SELENON (By similarity). In cardiac muscles, identified in a complex composed of FSD2, CMYA5 and RYR2 (By similarity).
Homotetramer. Can also form heterotetramers with RYR1 and RYR3 (By similarity). Interacts with FKBP1A and FKBP1B; these interactions may stabilize the channel in its closed state and prevent Ca(2+) leaks. Interacts with CALM and S100A1; these interactions regulate channel activity. Identified in a complex composed of RYR2, FKBP1B, PKA catalytic subunit, PRKAR2A, AKAP6, and the protein phosphatases PP2A and PP1. Interacts directly with FKBP1B, PKA, PP1 and PP2A. Interacts with SELENON (By similarity). In cardiac muscles, identified in a complex, composed of FSD2, CMYA5 and RYR2 (By similarity).
Homotetramer. Can also form heterotetramers with RYR1 and RYR3. Interacts with CALM and S100A1; these interactions regulate channel activity. Identified in a complex composed of RYR2, FKBP1B, PKA catalytic subunit, PRKAR2A, AKAP6, and the protein phosphatases PP2A and PP1. Interacts directly with FKBP1B, PKA, PP1 and PP2A (By similarity). Interacts with FKBP1A and FKBP1B; these interactions may stabilize the channel in its closed state and prevent Ca(2+) leaks. Interacts with SELENON (By similarity). Identified in a complex, composed of FSD2, CMYA5 and RYR2 (PubMed:28740084).
Homotetramer. Can also form heterotetramers with RYR1 and RYR3. Interacts with FKBP1A and FKBP1B; these interactions may stabilize the channel in its closed state and prevent Ca(2+) leaks. Interacts with CALM and S100A1; these interactions regulate channel activity. Identified in a complex composed of RYR2, FKBP1B, PKA catalytic subunit, PRKAR2A, AKAP6, and the protein phosphatases PP2A and PP1. Interacts directly with FKBP1B, PKA, PP1 and PP2A (By similarity). Interacts with SELENON (PubMed:18713863). In cardiac muscles, identified in a complex, composed of FSD2, CMYA5 and RYR2 (By similarity).
Homotetramer. Heterotetramer with RYR2. Interacts with CALM (By similarity). Interacts with FKBP1A. Interacts with SELENON (By similarity).
Homotetramer. Isoform 2 can form tetramers with isoform 1. Heterotetramer with RYR2. Interacts with FKBP1A. Interacts with CALM. Interacts with SELENON (By similarity).
Homotetramer. Isoform 2 can form tetramers with isoform 1. Heterotetramer with RYR2. Interacts with FKBP1A. Interacts with CALM. Interacts with SELENON (PubMed:18713863).
Homotetramer (PubMed:10097181, PubMed:15908964, PubMed:17027503, PubMed:18621707, PubMed:25470059, PubMed:25517095, PubMed:27662087, PubMed:27573175, PubMed:27468892). Can also form heterotetramers with RYR2 (PubMed:12213830). Identified in a complex composed of RYR1, PDE4D, PKA, FKBP1A and protein phosphatase 1 (PP1). Repeated very high-level exercise decreases interaction with PDE4D and protein phosphatase 1 (PP1) (By similarity). Interacts with CALM; CALM with bound calcium inhibits the RYR1 channel activity (PubMed:10601232, PubMed:11562475, PubMed:17027503). Interacts with S100A1 (By similarity). Interacts with FKBP1A; this stabilizes the closed conformation of the channel (PubMed:7669046, PubMed:10603943, PubMed:26245150, PubMed:25517095, PubMed:27468892). Interacts with CACNA1S; interaction with CACNA1S is important for activation of the RYR1 channel (PubMed:10388749). Interacts with CACNB1 (PubMed:21320436). Interacts with TRDN and ASPH; these interactions stimulate RYR1 channel activity (PubMed:9737879, PubMed:19398037). Interacts with SELENON (PubMed:18713863). Interacts with scorpion calcins (AC P0DPT1; AC P0DM30; AC A0A1L4BJ42; AC P59868; AC P60254; AC B8QG00; AC L0GBR1; AC P60252; AC P60253) (PubMed:27114612).
Homotetramer (PubMed:18003898). Can also form heterotetramers with RYR2 (By similarity). Identified in a complex composed of RYR1, PDE4D, PKA, FKBP1A and protein phosphatase 1 (PP1) (PubMed:18268335). Repeated very high-level exercise decreases interaction with PDE4D and protein phosphatase 1 (PP1) (PubMed:18268335). Interacts with CALM; CALM with bound calcium inhibits the RYR1 channel activity (By similarity). Interacts with S100A1 (By similarity). Interacts with FKBP1A; this stabilizes the closed conformation of the channel. Interacts with CACNA1S; interaction with CACNA1S is important for activation of the RYR1 channel. Interacts with CACNB1. Interacts with TRDN and ASPH; these interactions stimulate RYR1 channel activity. Interacts with SELENON (By similarity). Interacts with scorpion calcins (AC P0DPT1; AC P0DM30; AC A0A1L4BJ42; AC P59868; AC P60254; AC B8QG00; AC L0GBR1; AC P60252; AC P60253) (By similarity).
Homotetramer. Can also form heterotetramers with RYR2 (By similarity). Identified in a complex composed of RYR1, PDE4D, PKA, FKBP1A and protein phosphatase 1 (PP1). Repeated very high-level exercise decreases interaction with PDE4D and protein phosphatase 1 (PP1) (By similarity). Interacts with CALM; CALM with bound calcium inhibits the RYR1 channel activity (By similarity). Interacts with S100A1 (By similarity). Interacts with FKBP1A; this stabilizes the closed conformation of the channel. Interacts with CACNA1S; interaction with CACNA1S is important for activation of the RYR1 channel. Interacts with CACNB1. Interacts with TRDN and ASPH; these interactions stimulate RYR1 channel activity. Interacts with SELENON (By similarity). Interacts with scorpion calcins (AC P0DPT1; AC P0DM30; AC A0A1L4BJ42; AC P59868; AC P60254; AC B8QG00; AC L0GBR1; AC P60252; AC P60253) (By similarity).
Homotetramer. Can also form heterotetramers with RYR2 (By similarity). Identified in a complex composed of RYR1, PDE4D, PKA, FKBP1A and protein phosphatase 1 (PP1) (PubMed:18268335). Repeated very high-level exercise decreases interaction with PDE4D and protein phosphatase 1 (PP1) (PubMed:18268335). Interacts with CALM; CALM with bound calcium inhibits the RYR1 channel activity (PubMed:18650434). Interacts with S100A1 (PubMed:18650434). Interacts with FKBP1A; this stabilizes the closed conformation of the channel. Interacts with CACNA1S; interaction with CACNA1S is important for activation of the RYR1 channel. Interacts with CACNB1. Interacts with TRDN and ASPH; these interactions stimulate RYR1 channel activity. Interacts with SELENON (By similarity). Interacts with scorpion calcins (AC P0DPT1; AC P0DM30; AC A0A1L4BJ42; AC P59868; AC P60254; AC B8QG00; AC L0GBR1; AC P60252; AC P60253) (By similarity).
Homotetramer. Can also form heterotetramers with RYR3. Identified in a complex composed of RYR2, FKBP1B, PKA catalytic subunit, PRKAR2A, AKAP6, and the protein phosphatases PP2A and PP1. Interacts directly with FKBP1B, PKA, PP1 and PP2A (By similarity). Interacts with FKBP1A and FKBP1B; these interactions may stabilize the channel in its closed state and prevent Ca(2+) leaks. Interacts with CALM and S100A1; these interactions regulate channel activity. Interacts with SELENON (By similarity). In cardiac muscles, identified in a complex composed of FSD2, CMYA5 and RYR2 (By similarity).
Homotetramer. Can also form heterotetramers with RYR1 and RYR3 (By similarity). Interacts with FKBP1A and FKBP1B; these interactions may stabilize the channel in its closed state and prevent Ca(2+) leaks. Interacts with CALM and S100A1; these interactions regulate channel activity. Identified in a complex composed of RYR2, FKBP1B, PKA catalytic subunit, PRKAR2A, AKAP6, and the protein phosphatases PP2A and PP1. Interacts directly with FKBP1B, PKA, PP1 and PP2A. Interacts with SELENON (By similarity). In cardiac muscles, identified in a complex, composed of FSD2, CMYA5 and RYR2 (By similarity).
Homotetramer. Can also form heterotetramers with RYR1 and RYR3. Interacts with CALM and S100A1; these interactions regulate channel activity. Identified in a complex composed of RYR2, FKBP1B, PKA catalytic subunit, PRKAR2A, AKAP6, and the protein phosphatases PP2A and PP1. Interacts directly with FKBP1B, PKA, PP1 and PP2A (By similarity). Interacts with FKBP1A and FKBP1B; these interactions may stabilize the channel in its closed state and prevent Ca(2+) leaks. Interacts with SELENON (By similarity). Identified in a complex, composed of FSD2, CMYA5 and RYR2 (PubMed:28740084).
Homotetramer. Can also form heterotetramers with RYR1 and RYR3. Interacts with FKBP1A and FKBP1B; these interactions may stabilize the channel in its closed state and prevent Ca(2+) leaks. Interacts with CALM and S100A1; these interactions regulate channel activity. Identified in a complex composed of RYR2, FKBP1B, PKA catalytic subunit, PRKAR2A, AKAP6, and the protein phosphatases PP2A and PP1. Interacts directly with FKBP1B, PKA, PP1 and PP2A (By similarity). Interacts with SELENON (PubMed:18713863). In cardiac muscles, identified in a complex, composed of FSD2, CMYA5 and RYR2 (By similarity).
Homotetramer. Heterotetramer with RYR2. Interacts with CALM (By similarity). Interacts with FKBP1A. Interacts with SELENON (By similarity).
Homotetramer. Isoform 2 can form tetramers with isoform 1. Heterotetramer with RYR2. Interacts with FKBP1A. Interacts with CALM. Interacts with SELENON (By similarity).
Homotetramer. Isoform 2 can form tetramers with isoform 1. Heterotetramer with RYR2. Interacts with FKBP1A. Interacts with CALM. Interacts with SELENON (PubMed:18713863).
Homotetramer (PubMed:10097181, PubMed:15908964, PubMed:17027503, PubMed:18621707, PubMed:25470059, PubMed:25517095, PubMed:27662087, PubMed:27573175, PubMed:27468892). Can also form heterotetramers with RYR2 (PubMed:12213830). Identified in a complex composed of RYR1, PDE4D, PKA, FKBP1A and protein phosphatase 1 (PP1). Repeated very high-level exercise decreases interaction with PDE4D and protein phosphatase 1 (PP1) (By similarity). Interacts with CALM; CALM with bound calcium inhibits the RYR1 channel activity (PubMed:10601232, PubMed:11562475, PubMed:17027503). Interacts with S100A1 (By similarity). Interacts with FKBP1A; this stabilizes the closed conformation of the channel (PubMed:7669046, PubMed:10603943, PubMed:26245150, PubMed:25517095, PubMed:27468892). Interacts with CACNA1S; interaction with CACNA1S is important for activation of the RYR1 channel (PubMed:10388749). Interacts with CACNB1 (PubMed:21320436). Interacts with TRDN and ASPH; these interactions stimulate RYR1 channel activity (PubMed:9737879, PubMed:19398037). Interacts with SELENON (PubMed:18713863). Interacts with scorpion calcins (AC P0DPT1; AC P0DM30; AC A0A1L4BJ42; AC P59868; AC P60254; AC B8QG00; AC L0GBR1; AC P60252; AC P60253) (PubMed:27114612).
Homotetramer (PubMed:18003898). Can also form heterotetramers with RYR2 (By similarity). Identified in a complex composed of RYR1, PDE4D, PKA, FKBP1A and protein phosphatase 1 (PP1) (PubMed:18268335). Repeated very high-level exercise decreases interaction with PDE4D and protein phosphatase 1 (PP1) (PubMed:18268335). Interacts with CALM; CALM with bound calcium inhibits the RYR1 channel activity (By similarity). Interacts with S100A1 (By similarity). Interacts with FKBP1A; this stabilizes the closed conformation of the channel. Interacts with CACNA1S; interaction with CACNA1S is important for activation of the RYR1 channel. Interacts with CACNB1. Interacts with TRDN and ASPH; these interactions stimulate RYR1 channel activity. Interacts with SELENON (By similarity). Interacts with scorpion calcins (AC P0DPT1; AC P0DM30; AC A0A1L4BJ42; AC P59868; AC P60254; AC B8QG00; AC L0GBR1; AC P60252; AC P60253) (By similarity).
Homotetramer. Can also form heterotetramers with RYR2 (By similarity). Identified in a complex composed of RYR1, PDE4D, PKA, FKBP1A and protein phosphatase 1 (PP1). Repeated very high-level exercise decreases interaction with PDE4D and protein phosphatase 1 (PP1) (By similarity). Interacts with CALM; CALM with bound calcium inhibits the RYR1 channel activity (By similarity). Interacts with S100A1 (By similarity). Interacts with FKBP1A; this stabilizes the closed conformation of the channel. Interacts with CACNA1S; interaction with CACNA1S is important for activation of the RYR1 channel. Interacts with CACNB1. Interacts with TRDN and ASPH; these interactions stimulate RYR1 channel activity. Interacts with SELENON (By similarity). Interacts with scorpion calcins (AC P0DPT1; AC P0DM30; AC A0A1L4BJ42; AC P59868; AC P60254; AC B8QG00; AC L0GBR1; AC P60252; AC P60253) (By similarity).
Homotetramer. Can also form heterotetramers with RYR2 (By similarity). Identified in a complex composed of RYR1, PDE4D, PKA, FKBP1A and protein phosphatase 1 (PP1) (PubMed:18268335). Repeated very high-level exercise decreases interaction with PDE4D and protein phosphatase 1 (PP1) (PubMed:18268335). Interacts with CALM; CALM with bound calcium inhibits the RYR1 channel activity (PubMed:18650434). Interacts with S100A1 (PubMed:18650434). Interacts with FKBP1A; this stabilizes the closed conformation of the channel. Interacts with CACNA1S; interaction with CACNA1S is important for activation of the RYR1 channel. Interacts with CACNB1. Interacts with TRDN and ASPH; these interactions stimulate RYR1 channel activity. Interacts with SELENON (By similarity). Interacts with scorpion calcins (AC P0DPT1; AC P0DM30; AC A0A1L4BJ42; AC P59868; AC P60254; AC B8QG00; AC L0GBR1; AC P60252; AC P60253) (By similarity).
Miscellaneous
Channel activity is modulated by the alkaloid ryanodine that binds to the open Ca-release channel with high affinity and maintains the channel in an open conformation. The calcium release channel is modulated by calcium ions, magnesium ions, ATP and calmodulin (By similarity).
Channel activity is modulated by the alkaloid ryanodine that binds to the open calcium-release channel with high affinity. At low concentrations, ryanodine maintains the channel in an open conformation. High ryanodine concentrations inhibit channel activity. Channel activity is regulated by calmodulin (CALM). The calcium release is activated by elevated cytoplasmic calcium levels in the micromolar range, by caffeine and adenine nucleotides, such as AMP and ATP. Inhibited by Mg(2+) and ruthenium red (By similarity).
Channel activity is modulated by the alkaloid ryanodine that binds to the open calcium-release channel with high affinity. At low concentrations, ryanodine maintains the channel in an open conformation. High ryanodine concentrations inhibit channel activity. Channel activity is regulated by calmodulin (CALM). The calcium release is activated by elevated cytoplasmic calcium levels in the micromolar range, by caffeine and adenine nucleotides, such as AMP and ATP. Inhibited by Mg(2+) and ruthenium red.
Coexpression of normal and mutant Thr-4897 RYR1 in a 1:1 ratio produces RYR1 channels with normal halothane and caffeine sensitivities, but maximal levels of Ca(2+) release are reduced by 67%. Binding of [3H]ryanodine indicates that the heterozygous channel is activated by Ca(2+) concentrations 4-fold lower than normal. Single-cell analysis of cotransfected cells shows a significantly increased resting cytoplasmic Ca(2+) level and a significantly reduced luminal Ca(2+) level. These data indicated a leaky channel, possibly caused by a reduction in the Ca(2+) concentration required for channel activation.
Coexpression of normal and mutant Thr-4898 RYR1 in a 1:1 ratio produces RYR1 channels with normal halothane and caffeine sensitivities, but maximal levels of Ca(2+) release are reduced by 67%. Binding of [3H]ryanodine indicates that the heterozygous channel is activated by Ca(2+) concentrations 4-fold lower than normal. Single-cell analysis of cotransfected cells shows a significantly increased resting cytoplasmic Ca(2+) level and a significantly reduced luminal Ca(2+) level. These data indicated a leaky channel, possibly caused by a reduction in the Ca(2+) concentration required for channel activation. Comparison with 2 other coexpressed mutant/normal channels suggests that the Thr-4898 mutation produces one of the most abnormal RYR1 channels that has been investigated, and this level of abnormality is reflected in the severe and penetrant phenotype of affected CCD individuals.
Channel activity is modulated by the alkaloid ryanodine that binds to the open calcium-release channel with high affinity. At low concentrations, ryanodine maintains the channel in an open conformation. High ryanodine concentrations inhibit channel activity. Channel activity is regulated by calmodulin (CALM). The calcium release is activated by elevated cytoplasmic calcium levels in the micromolar range, by caffeine and adenine nucleotides, such as AMP and ATP. Inhibited by Mg(2+) and ruthenium red (By similarity).
Channel activity is modulated by the alkaloid ryanodine that binds to the open calcium-release channel with high affinity. At low concentrations, ryanodine maintains the channel in an open conformation. High ryanodine concentrations inhibit channel activity. Channel activity is regulated by calmodulin (CALM). The calcium release is activated by elevated cytoplasmic calcium levels in the micromolar range, by caffeine and adenine nucleotides, such as AMP and ATP. Inhibited by Mg(2+) and ruthenium red.
Coexpression of normal and mutant Thr-4897 RYR1 in a 1:1 ratio produces RYR1 channels with normal halothane and caffeine sensitivities, but maximal levels of Ca(2+) release are reduced by 67%. Binding of [3H]ryanodine indicates that the heterozygous channel is activated by Ca(2+) concentrations 4-fold lower than normal. Single-cell analysis of cotransfected cells shows a significantly increased resting cytoplasmic Ca(2+) level and a significantly reduced luminal Ca(2+) level. These data indicated a leaky channel, possibly caused by a reduction in the Ca(2+) concentration required for channel activation.
Coexpression of normal and mutant Thr-4898 RYR1 in a 1:1 ratio produces RYR1 channels with normal halothane and caffeine sensitivities, but maximal levels of Ca(2+) release are reduced by 67%. Binding of [3H]ryanodine indicates that the heterozygous channel is activated by Ca(2+) concentrations 4-fold lower than normal. Single-cell analysis of cotransfected cells shows a significantly increased resting cytoplasmic Ca(2+) level and a significantly reduced luminal Ca(2+) level. These data indicated a leaky channel, possibly caused by a reduction in the Ca(2+) concentration required for channel activation. Comparison with 2 other coexpressed mutant/normal channels suggests that the Thr-4898 mutation produces one of the most abnormal RYR1 channels that has been investigated, and this level of abnormality is reflected in the severe and penetrant phenotype of affected CCD individuals.
Similarity
Belongs to the ryanodine receptor (TC 1.A.3.1) family.
Belongs to the ryanodine receptor (TC 1.A.3.1) family. RYR2 subfamily.
Belongs to the ryanodine receptor (TC 1.A.3.1) family. RYR3 subfamily.
Belongs to the ryanodine receptor (TC 1.A.3.1) family. RYR1 subfamily.
Belongs to the ryanodine receptor (TC 1.A.3.1) family. RYR2 subfamily.
Belongs to the ryanodine receptor (TC 1.A.3.1) family. RYR3 subfamily.
Belongs to the ryanodine receptor (TC 1.A.3.1) family. RYR1 subfamily.
Keywords
Alternative splicing
Calcium
Calcium channel
Calcium transport
Complete proteome
Developmental protein
Ion channel
Ion transport
Ligand-gated ion channel
Membrane
Phosphoprotein
Receptor
Reference proteome
Repeat
Sarcoplasmic reticulum
Transmembrane
Transmembrane helix
Transport
3D-structure
Calmodulin-binding
Cardiomyopathy
Coiled coil
Disease mutation
Polymorphism
Endoplasmic reticulum
Microsome
ATP-binding
Direct protein sequencing
Metal-binding
Nucleotide-binding
S-nitrosylation
Feature
chain Ryanodine receptor
splice variant In isoform C and isoform D.
sequence variant Found in a patient with short-coupled polymorphic ventricular tachycardia at rest; unknown pathological significance; no effect on cytosolic Ca(2+) activation.
splice variant In isoform C and isoform D.
sequence variant Found in a patient with short-coupled polymorphic ventricular tachycardia at rest; unknown pathological significance; no effect on cytosolic Ca(2+) activation.
Uniprot
Pubmed
8276118
10731132
12537572
1338312
10811919
18327897
+ More
20445169 20471962 18755143 20431056 22673903 18650434 8809036 11159936 16710414 9148749 9607712 10830164 20056922 11805843 19482609 21472222 20961976 25372681 11208676 11157710 12093772 12106942 14571276 15466642 15046072 15046073 15544015 16188589 25356899 17984046 24793461 25463374 26405799 27733687 10473538 19468303 9628868 7876312 7621815 17693412 21183079 21098440 20214899 28740084 19913485 21645850 22705209 23978697 26245150 2380170 8841406 1645727 12213830 18713863 9395096 9515741 16572171 7523185 8276408 7556644 12354756 22100703 8702664 16141072 7635066 9582272 11717163 11500519 17596299 19503748 1330694 9305876 12471029 9614063 10358090 16176801 16274254 2725677 2298749 10601232 3722165 8380342 7669046 10603943 9737879 10388749 10097181 11562475 12486242 12732639 19398037 21320436 22036948 27114612 15908964 17027503 18621707 19541610 21048710 22913516 23422674 25370123 25470059 25517095 27662087 27573175 27468892 18003898 7515481 15489334 7724570 18268335 21156754 15057822 10444400 11316255 16641100 1329581 8288238 2174405 1354642 8220422 8661021 15057824 1639409 1774074 7751854 18318008 22752422 8220423 7829078 8012359 7849712 7881417 9066328 9389851 9138151 9497245 9450902 10484775 10051009 10823104 10888602 10612851 11113224 11389482 11575529 11709545 11741831 11241852 11525881 12059893 12411788 12112081 12066726 12208234 12123492 11928716 12136074 12883402 14670767 12937085 12709367 12566385 12719381 12565913 15448513 14732627 14985404 15221887 16163667 16380615 17204054 17226826 18312400 18253926 19191329 19685112 20142353 20583297 20681998 21674524 24013571 23558838 24561095 26381711 26115329 27586648 26631338 27616680 27234031
20445169 20471962 18755143 20431056 22673903 18650434 8809036 11159936 16710414 9148749 9607712 10830164 20056922 11805843 19482609 21472222 20961976 25372681 11208676 11157710 12093772 12106942 14571276 15466642 15046072 15046073 15544015 16188589 25356899 17984046 24793461 25463374 26405799 27733687 10473538 19468303 9628868 7876312 7621815 17693412 21183079 21098440 20214899 28740084 19913485 21645850 22705209 23978697 26245150 2380170 8841406 1645727 12213830 18713863 9395096 9515741 16572171 7523185 8276408 7556644 12354756 22100703 8702664 16141072 7635066 9582272 11717163 11500519 17596299 19503748 1330694 9305876 12471029 9614063 10358090 16176801 16274254 2725677 2298749 10601232 3722165 8380342 7669046 10603943 9737879 10388749 10097181 11562475 12486242 12732639 19398037 21320436 22036948 27114612 15908964 17027503 18621707 19541610 21048710 22913516 23422674 25370123 25470059 25517095 27662087 27573175 27468892 18003898 7515481 15489334 7724570 18268335 21156754 15057822 10444400 11316255 16641100 1329581 8288238 2174405 1354642 8220422 8661021 15057824 1639409 1774074 7751854 18318008 22752422 8220423 7829078 8012359 7849712 7881417 9066328 9389851 9138151 9497245 9450902 10484775 10051009 10823104 10888602 10612851 11113224 11389482 11575529 11709545 11741831 11241852 11525881 12059893 12411788 12112081 12066726 12208234 12123492 11928716 12136074 12883402 14670767 12937085 12709367 12566385 12719381 12565913 15448513 14732627 14985404 15221887 16163667 16380615 17204054 17226826 18312400 18253926 19191329 19685112 20142353 20583297 20681998 21674524 24013571 23558838 24561095 26381711 26115329 27586648 26631338 27616680 27234031
EMBL
D17389
AE013599
Z18536
AAF59036.2
EU346200
AB204523
+ More
X98330 AJ300340 AJ300341 AJ300342 AJ300343 AJ300347 AJ300349 AJ300351 AJ300353 AJ300355 AJ300364 AJ300363 AJ300362 AJ300361 AJ300360 AJ300359 AJ300358 AJ300357 AJ300356 AJ300373 AJ300372 AJ300371 AJ300370 AJ300369 AJ300368 AJ300367 AJ300366 AJ300365 AJ300382 AJ300381 AJ300380 AJ300379 AJ300378 AJ300377 AJ300376 AJ300375 AJ300374 AJ300399 AJ300398 AJ300397 AJ300396 AJ300395 AJ300394 AJ300393 AJ300392 AJ300391 AJ300416 AJ300415 AJ300414 AJ300413 AJ300412 AJ300411 AJ300410 AJ300409 AJ300408 AJ300433 AJ300432 AJ300431 AJ300430 AJ300429 AJ300428 AJ300427 AJ300426 AJ300425 AJ300444 AJ300443 AJ300442 AJ300441 AJ300440 AJ300439 AJ300438 AJ300437 AJ300436 AJ300435 AJ300434 AJ300424 AJ300423 AJ300422 AJ300421 AJ300420 AJ300419 AJ300418 AJ300417 AJ300407 AJ300406 AJ300405 AJ300404 AJ300403 AJ300402 AJ300401 AJ300400 AJ300390 AJ300389 AJ300388 AJ300387 AJ300386 AJ300385 AJ300384 AJ300383 AJ300354 AJ300352 AJ300350 AJ300348 AJ300346 AJ300345 AJ300344 AL365332 AL356773 AL359924 AL391809 AL442065 AL445473 AL513130 Y08218 X91869 AJ002511 AF295105 AC131329 AC159208 CT010468 CT572985 AB012003 X83933 D38217 M59743 AB001025 AJ001515 AC010809 AC011938 AC055874 AC067793 AC087638 AJ002512 X74269 X74270 AL929348 AL672250 AL691423 AL732316 BX649564 D84237 AF111166 AK132464 X83934 U23756 D38218 X68650 X15209 X15750 AY268935 AC164564 AC165142 D21798 D21796 D21797 AJ308737 BC051248 BC055487 X83932 U23754 D38216 AF112256 AF130879 AF011788 X62880 X68247 X69465 M32501 J05200 U48508 U48449 U48450 U48451 U48452 U48453 U48454 U48455 U48456 U48457 U48458 U48459 U48460 U48461 U48462 U48463 U48464 U48465 U48466 U48467 U48468 U48469 U48470 U48471 U48472 U48473 U48474 U48475 U48476 U48477 U48478 U48479 U48480 U48481 U48482 U48483 U48484 U48485 U48486 U48487 U48488 U48489 U48490 U48491 U48492 U48493 U48494 U48495 U48496 U48497 U48498 U48499 U48500 U48501 U48502 U48503 U48504 U48505 U48506 U48507 AC067969 AC005933 AC011469 M91455 S78717 S77392
X98330 AJ300340 AJ300341 AJ300342 AJ300343 AJ300347 AJ300349 AJ300351 AJ300353 AJ300355 AJ300364 AJ300363 AJ300362 AJ300361 AJ300360 AJ300359 AJ300358 AJ300357 AJ300356 AJ300373 AJ300372 AJ300371 AJ300370 AJ300369 AJ300368 AJ300367 AJ300366 AJ300365 AJ300382 AJ300381 AJ300380 AJ300379 AJ300378 AJ300377 AJ300376 AJ300375 AJ300374 AJ300399 AJ300398 AJ300397 AJ300396 AJ300395 AJ300394 AJ300393 AJ300392 AJ300391 AJ300416 AJ300415 AJ300414 AJ300413 AJ300412 AJ300411 AJ300410 AJ300409 AJ300408 AJ300433 AJ300432 AJ300431 AJ300430 AJ300429 AJ300428 AJ300427 AJ300426 AJ300425 AJ300444 AJ300443 AJ300442 AJ300441 AJ300440 AJ300439 AJ300438 AJ300437 AJ300436 AJ300435 AJ300434 AJ300424 AJ300423 AJ300422 AJ300421 AJ300420 AJ300419 AJ300418 AJ300417 AJ300407 AJ300406 AJ300405 AJ300404 AJ300403 AJ300402 AJ300401 AJ300400 AJ300390 AJ300389 AJ300388 AJ300387 AJ300386 AJ300385 AJ300384 AJ300383 AJ300354 AJ300352 AJ300350 AJ300348 AJ300346 AJ300345 AJ300344 AL365332 AL356773 AL359924 AL391809 AL442065 AL445473 AL513130 Y08218 X91869 AJ002511 AF295105 AC131329 AC159208 CT010468 CT572985 AB012003 X83933 D38217 M59743 AB001025 AJ001515 AC010809 AC011938 AC055874 AC067793 AC087638 AJ002512 X74269 X74270 AL929348 AL672250 AL691423 AL732316 BX649564 D84237 AF111166 AK132464 X83934 U23756 D38218 X68650 X15209 X15750 AY268935 AC164564 AC165142 D21798 D21796 D21797 AJ308737 BC051248 BC055487 X83932 U23754 D38216 AF112256 AF130879 AF011788 X62880 X68247 X69465 M32501 J05200 U48508 U48449 U48450 U48451 U48452 U48453 U48454 U48455 U48456 U48457 U48458 U48459 U48460 U48461 U48462 U48463 U48464 U48465 U48466 U48467 U48468 U48469 U48470 U48471 U48472 U48473 U48474 U48475 U48476 U48477 U48478 U48479 U48480 U48481 U48482 U48483 U48484 U48485 U48486 U48487 U48488 U48489 U48490 U48491 U48492 U48493 U48494 U48495 U48496 U48497 U48498 U48499 U48500 U48501 U48502 U48503 U48504 U48505 U48506 U48507 AC067969 AC005933 AC011469 M91455 S78717 S77392
Proteomes
PRIDE
Pfam
Interpro
IPR036300
MIR_dom_sf
+ More
IPR001870 B30.2/SPRY
IPR035761 SPRY1_RyR
IPR009460 Ryanrecept_TM4-6
IPR014821 Ins145_P3_rcpt
IPR000699 RIH_dom
IPR013662 RIH_assoc-dom
IPR035910 RyR/IP3R_RIH_dom_sf
IPR035762 SPRY3_RyR
IPR011992 EF-hand-dom_pair
IPR013320 ConA-like_dom_sf
IPR005821 Ion_trans_dom
IPR013333 Ryan_recept
IPR003877 SPRY_dom
IPR016093 MIR_motif
IPR003032 Ryanodine_rcpt
IPR035764 SPRY2_RyR
IPR002048 EF_hand_dom
IPR016024 ARM-type_fold
IPR033215 RyR1
IPR001870 B30.2/SPRY
IPR035761 SPRY1_RyR
IPR009460 Ryanrecept_TM4-6
IPR014821 Ins145_P3_rcpt
IPR000699 RIH_dom
IPR013662 RIH_assoc-dom
IPR035910 RyR/IP3R_RIH_dom_sf
IPR035762 SPRY3_RyR
IPR011992 EF-hand-dom_pair
IPR013320 ConA-like_dom_sf
IPR005821 Ion_trans_dom
IPR013333 Ryan_recept
IPR003877 SPRY_dom
IPR016093 MIR_motif
IPR003032 Ryanodine_rcpt
IPR035764 SPRY2_RyR
IPR002048 EF_hand_dom
IPR016024 ARM-type_fold
IPR033215 RyR1
SUPFAM
ProteinModelPortal
PDB
5GOA
E-value=0,
Score=6409
Ontologies
GO
GO:0007275
GO:0005219
GO:0006936
GO:0060047
GO:0035206
GO:0016021
GO:0006816
GO:0033017
GO:0072347
GO:0005886
GO:0031672
GO:0097110
GO:0005262
GO:0019722
GO:0005509
GO:0042383
GO:0006874
GO:0043005
GO:0016529
GO:0070296
GO:0048763
GO:0005783
GO:0031234
GO:0034704
GO:0051592
GO:0042493
GO:0015278
GO:0032026
GO:0005790
GO:0007584
GO:0005737
GO:0051480
GO:0034220
GO:0051481
GO:0003143
GO:0070588
GO:0005635
GO:0051209
GO:0071313
GO:0030659
GO:0071421
GO:0030018
GO:0005516
GO:0032991
GO:0014808
GO:1903779
GO:0014850
GO:0051284
GO:0014701
GO:0060402
GO:0003300
GO:0098907
GO:0071872
GO:0034237
GO:0098735
GO:0035584
GO:0055117
GO:0098910
GO:0034236
GO:0060070
GO:0035994
GO:0001666
GO:0031000
GO:1901896
GO:0097050
GO:0005513
GO:0098904
GO:0086005
GO:0019899
GO:0086064
GO:0010460
GO:0043924
GO:0060048
GO:0010882
GO:0042802
GO:0016020
GO:0002027
GO:0044325
GO:0072599
GO:0010881
GO:0051775
GO:0098911
GO:0030509
GO:0043621
GO:0086029
GO:0003220
GO:0060401
GO:0019901
GO:0030017
GO:0097159
GO:0043231
GO:0008144
GO:0071318
GO:0048471
GO:0051289
GO:0071277
GO:0071286
GO:0030314
GO:0006941
GO:0048741
GO:1990425
GO:0005524
GO:0043931
GO:0003151
GO:0035381
GO:0043588
GO:0015643
GO:0031301
GO:0097718
GO:0005245
GO:0014802
GO:0006937
GO:0005938
GO:0030315
GO:0031674
GO:0002020
GO:0032355
GO:0070062
GO:0005887
GO:0005515
GO:0005507
GO:0004713
GO:0016491
GO:0015930
PANTHER
Topology
Subcellular location
Sarcoplasmic reticulum membrane
The number of predicted transmembrane domains varies between orthologs, but both N-terminus and C-terminus seem to be cytoplasmic. With evidence from 1 publications.
Membrane The number of predicted transmembrane domains varies between orthologs, but both N-terminus and C-terminus seem to be cytoplasmic. With evidence from 1 publications.
Sarcoplasmic reticulum The number of predicted transmembrane domains varies between orthologs, but both N-terminus and C-terminus seem to be cytoplasmic. With evidence from 3 publications.
Microsome membrane The number of predicted transmembrane domains varies between orthologs, but both N-terminus and C-terminus seem to be cytoplasmic. With evidence from 2 publications.
Membrane The number of predicted transmembrane domains varies between orthologs, but both N-terminus and C-terminus seem to be cytoplasmic. With evidence from 1 publications.
Sarcoplasmic reticulum The number of predicted transmembrane domains varies between orthologs, but both N-terminus and C-terminus seem to be cytoplasmic. With evidence from 3 publications.
Microsome membrane The number of predicted transmembrane domains varies between orthologs, but both N-terminus and C-terminus seem to be cytoplasmic. With evidence from 2 publications.
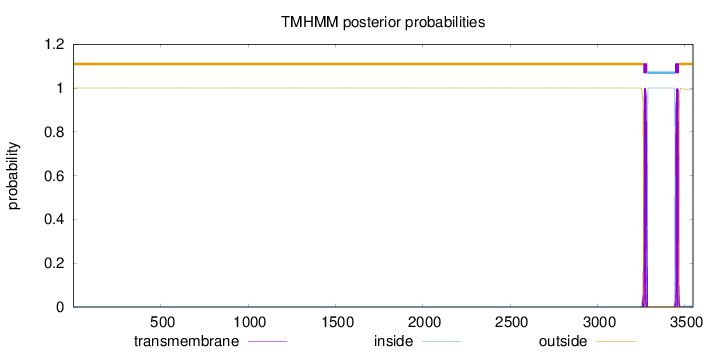
Length:
3547
Number of predicted TMHs:
2
Exp number of AAs in TMHs:
44.06194
Exp number, first 60 AAs:
0
Total prob of N-in:
0.00001
outside
1 - 3263
TMhelix
3264 - 3286
inside
3287 - 3444
TMhelix
3445 - 3467
outside
3468 - 3547
Population Genetic Test Statistics
Pi
267.648687
Theta
177.328796
Tajima's D
1.672083
CLR
0.109145
CSRT
0.822308884555772
Interpretation
Uncertain
